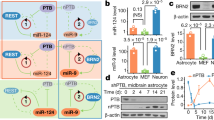
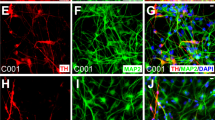
Abstract
Cell replacement therapies for neurodegenerative disease have focused on transplantation of the cell types affected by the pathological process. Here we describe an alternative strategy for Parkinson's disease in which dopamine neurons are generated by direct conversion of astrocytes. Using three transcription factors, NEUROD1, ASCL1 and LMX1A, and the microRNA miR218, collectively designated NeAL218, we reprogram human astrocytes in vitro, and mouse astrocytes in vivo, into induced dopamine neurons (iDANs). Reprogramming efficiency in vitro is improved by small molecules that promote chromatin remodeling and activate the TGFβ, Shh and Wnt signaling pathways. The reprogramming efficiency of human astrocytes reaches up to 16%, resulting in iDANs with appropriate midbrain markers and excitability. In a mouse model of Parkinson's disease, NeAL218 alone reprograms adult striatal astrocytes into iDANs that are excitable and correct some aspects of motor behavior in vivo, including gait impairments. With further optimization, this approach may enable clinical therapies for Parkinson's disease by delivery of genes rather than cells.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
Change history
19 April 2017
In the version of this article initially published, in the paragraph before the Discussion, the sentence that read in part “…the very high levels of DA that are required in the synapses to induce circling behavior…” should have read “…the very high levels of DA that are required in the synapses to reduce circling behavior…” The error has been corrected for the print, PDF and HTML versions of this article.
References
DeMaagd, G. & Philip, A. Parkinson's disease and its management. Part 2: Introduction to the pharmacotherapy of parkinson's disease, with a focus on the use of dopaminergic agents. P&T 40, 591–600 (2015).
Brundin, P. et al. Intracerebral grafting of dopamine neurons. Experimental basis for clinical trials in patients with Parkinson's disease. Ann. NY Acad. Sci. 495, 473–496 (1987).
Lindvall, O. et al. Fetal dopamine-rich mesencephalic grafts in Parkinson's disease. Lancet 2, 1483–1484 (1988).
Arenas, E. Towards stem cell replacement therapies for Parkinson's disease. Biochem. Biophys. Res. Commun. 396, 152–156 (2010).
Arenas, E., Denham, M. & Villaescusa, J.C. How to make a midbrain dopaminergic neuron. Development 142, 1918–1936 (2015).
Takahashi, K. & Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006).
Vierbuchen, T. et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463, 1035–1041 (2010).
Ladewig, J., Koch, P. & Brustle, O. Leveling Waddington: the emergence of direct reprogramming and the loss of cell fate hierarchies. Nat. Rev. Mol. Cell Biol. 14, 225–236 (2013).
Chen, G. et al. In vivo reprogramming for brain and spinal cord repair(1,2,3). eNeuro. 2, e0106–15.2015 (2015).
Caiazzo, M. et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 476, 224–227 (2011).
Kim, J. et al. Functional integration of dopaminergic neurons directly converted from mouse fibroblasts. Cell Stem Cell 9, 413–419 (2011).
Liu, X. et al. Direct reprogramming of human fibroblasts into dopaminergic neuron-like cells. Cell Res. 22, 321–332 (2012).
Pfisterer, U. et al. Direct conversion of human fibroblasts to dopaminergic neurons. Proc. Natl. Acad. Sci. USA 108, 10343–10348 (2011).
Addis, R.C. et al. Efficient conversion of astrocytes to functional midbrain dopaminergic neurons using a single polycistronic vector. PLoS One 6, e28719 (2011).
Heins, N. et al. Glial cells generate neurons: the role of the transcription factor Pax6. Nat. Neurosci. 5, 308–315 (2002).
Heinrich, C. et al. Directing astroglia from the cerebral cortex into subtype specific functional neurons. PLoS Biol. 8, e1000373 (2010).
Heinrich, C. et al. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nat. Protoc. 6, 214–228 (2011).
Torper, O. et al. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA 110, 7038–7043 (2013).
Su, Z., Niu, W., Liu, M.L., Zou, Y. & Zhang, C.L. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat. Commun. 5, 3338 (2014).
Guo, Z. et al. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury and in an Alzheimer's disease model. Cell Stem Cell 14, 188–202 (2014).
Heinrich, C. et al. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports 3, 1000–1014 (2014).
Torper, O. et al. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 12, 474–481 (2015).
Gascón, S. et al. Identification and successful negotiation of a metabolic checkpoint in direct neuronal reprogramming. Cell Stem Cell 18, 396–409 (2016).
Brambrink, T. et al. Sequential expression of pluripotency markers during direct reprogramming of mouse somatic cells. Cell Stem Cell 2, 151–159 (2008).
Esteban, M.A. et al. Vitamin C enhances the generation of mouse and human induced pluripotent stem cells. Cell Stem Cell 6, 71–79 (2010).
Chambers, S.M. et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat. Biotechnol. 27, 275–280 (2009).
Wernig, M. et al. Tau EGFP embryonic stem cells: an efficient tool for neuronal lineage selection and transplantation. J. Neurosci. Res. 69, 918–924 (2002).
Kriks, S. et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson's disease. Nature 480, 547–551 (2011).
Pang, Z.P. et al. Induction of human neuronal cells by defined transcription factors. Nature 476, 220–223 (2011).
Huang, T., Liu, Y., Huang, M., Zhao, X. & Cheng, L. Wnt1-cre-mediated conditional loss of Dicer results in malformation of the midbrain and cerebellum and failure of neural crest and dopaminergic differentiation in mice. J. Mol. Cell Biol. 2, 152–163 (2010).
Huangfu, D. et al. Induction of pluripotent stem cells by defined factors is greatly improved by small-molecule compounds. Nat. Biotechnol. 26, 795–797 (2008).
Pennarossa, G. et al. Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proc. Natl. Acad. Sci. USA 110, 8948–8953 (2013).
Chung, T.L. et al. Vitamin C promotes widespread yet specific DNA demethylation of the epigenome in human embryonic stem cells. Stem Cells 28, 1848–1855 (2010).
Liu, X. et al. Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT-MET mechanism for optimal reprogramming. Nat. Cell Biol. 15, 829–838 (2013).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580.e19 (2016).
Koyano-Nakagawa, N., Kim, J., Anderson, D. & Kintner, C. Hes6 acts in a positive feedback loop with the neurogenins to promote neuronal differentiation. Development 127, 4203–4216 (2000).
Jhas, S. et al. Hes6 inhibits astrocyte differentiation and promotes neurogenesis through different mechanisms. J. Neurosci. 26, 11061–11071 (2006).
Dillon-Carter, O., Conejero, C., Tornatore, C., Poltorak, M. & Freed, W.J. N18-RE-105 cells: differentiation and activation of p53 in response to glutamate and adriamycin is blocked by SV40 large T antigen tsA58. Cell Tissue Res. 291, 191–205 (1998).
Lundblad, M., Picconi, B., Lindgren, H. & Cenci, M.A. A model of L-DOPA-induced dyskinesia in 6-hydroxydopamine lesioned mice: relation to motor and cellular parameters of nigrostriatal function. Neurobiol. Dis. 16, 110–123 (2004).
Darmopil, S., Muñetón-Gómez, V.C., de Ceballos, M.L., Bernson, M. & Moratalla, R. Tyrosine hydroxylase cells appearing in the mouse striatum after dopamine denervation are likely to be projection neurones regulated by L-DOPA. Eur. J. Neurosci. 27, 580–592 (2008).
Masuda, M. et al. Postnatal development of tyrosine hydroxylase mRNA-expressing neurons in mouse neostriatum. Eur. J. Neurosci. 34, 1355–1367 (2011).
Hu, G. et al. New fluorescent substrate enables quantitative and high-throughput examination of vesicular monoamine transporter 2 (VMAT2). ACS Chem. Biol. 8, 1947–1954 (2013).
Freyberg, Z. et al. Mechanisms of amphetamine action illuminated through optical monitoring of dopamine synaptic vesicles in Drosophila brain. Nat. Commun. 7, 10652 (2016).
Brooks, S.P. & Dunnett, S.B. Tests to assess motor phenotype in mice: a user's guide. Nat. Rev. Neurosci. 10, 519–529 (2009).
Bagga, V., Dunnett, S.B. & Fricker, R.A. The 6-OHDA mouse model of Parkinson's disease - Terminal striatal lesions provide a superior measure of neuronal loss and replacement than median forebrain bundle lesions. Behav. Brain Res. 288, 107–117 (2015).
Fasano, A., Aquino, C.C., Krauss, J.K., Honey, C.R. & Bloem, B.R. Axial disability and deep brain stimulation in patients with Parkinson disease. Nat. Rev. Neurol. 11, 98–110 (2015).
Kurz, M.J. et al. A chronic mouse model of Parkinson's disease has a reduced gait pattern certainty. Neurosci. Lett. 429, 39–42 (2007).
Bonito-Oliva, A., Masini, D. & Fisone, G. A mouse model of non-motor symptoms in Parkinson's disease: focus on pharmacological interventions targeting affective dysfunctions. Front. Behav. Neurosci. 8, 290 (2014).
Amende, I. et al. Gait dynamics in mouse models of Parkinson's disease and Huntington's disease. J. Neuroeng. Rehabil. 2, 20 (2005).
Wang, H., Li, X., Gao, S., Sun, X. & Fang, H. Transdifferentiation via transcription factors or microRNAs: current status and perspective. Differentiation 90, 69–76 (2015).
Dickson, D.W. Parkinson's disease and parkinsonism: neuropathology. Cold Spring Harb. Perspect. Med. 2, a009258 (2012).
Zeisel, A. et al. Brain structure. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science 347, 1138–1142 (2015).
Love, M.I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014).
Wang, J., Lin, W., Popko, B. & Campbell, I.L. Inducible production of interferon-gamma in the developing brain causes cerebellar dysplasia with activation of the Sonic hedgehog pathway. Mol. Cell. Neurosci. 27, 489–496 (2004).
Ekstrand, M.I. et al. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc. Natl. Acad. Sci. USA 104, 1325–1330 (2007).
Acknowledgements
We thank the members of the Arenas laboratory for help and suggestions; J. Söderlund and A. Nanni for technical and secretarial assistance; and the SciLife National Genomic Infrastructure (Stockholm) for RNA sequencing. This work was supported by grants from the Swedish Research Council (VR: DBRM, 2011-3116/3318 and 2016-01526), Swedish Foundation for Strategic Research (SRL), EU (NeuroStemcellRepair and DDPDgenes), Karolinska Institutet, Strat Regen, Hjärnfonden (FO2013:0108, FO2015:0202) and Cancerfonden (CAN 2016/572) to E.A.; VR (2012-13482 and 2015-02886), StratNeuro, Parkinsonfonden, Hjärnfonden and KI/NIH to G.F.; VR (2013-3080), EU (PAINCAGE), Hjärnfonden, NovoNordisk Foundation and the European Research Council (“Secret Cells”) to T.H.; and New York Stem Cell Foundation, NIH and CIRM to M.W. Support to P.R.d.V.C. was provided by VR (524-2011-962) and EMBO (ALTF583-2011); to R.A.R. by EMBO (ALTF596-2014) and Marie Curie (EMBOCOFUND2012, GA-2012-600394); to D.M. by KI and by the Brasilian Ministry of Education (CAPES) and to E.M.-M. by the Spanish Ministry of Education (José Castillejo). The authors acknowledge support from Science for Life Laboratory, the Knut and Alice Wallenberg Foundation, the National Genomics Infrastructure funded by the Swedish Research Council, and Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure.
Author information
Authors and Affiliations
Contributions
E.A. and P.R.d.V.C. conceived the experiments and wrote the manuscript; P.R.d.V.C., R.A.R., G.S., D.M., E.M.-M., E.M.T., G.L.M., M.F., C.P., Y.-H.N. and S.P.S. performed the experiments; S. L., M.W., T.H., G.F. and E.A. provided expertise and funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
Supplementary Figure 1 Characterization of hIA cultures and midbrain markers after iDAN reprogramming.
a. Uninfected hIAs do not spontaneously become TH+ in MP media at day 17. b. hIAs infected with GFP show a high infection efficiency in MP media at day 17 c. Gene expression analysis by real-time q-PCR revealed an increase in multiple mDA markers in hIA cultures treated with AL-MP, AL218-MP or NeAL218-MP at day 10. Data are shown as fold increase over control cells. * p<0.05 and ** p<0.01, n≥3, n.d.: not detectable
Supplementary Figure 2 Properties of hIA-derived iDANsNeAL218-RTMP cells.
a. Percentage of hIAs that become TH+ after infection with NeAL218 in RTMP conditions at day 4(n=3) and day 10(n=3). b. hIAs infected with NeAL218 in RTMP conditions at day 4 (scale bar 50μm) c. Illustration of the experiment for doxycycline withdrawal. d. hIAs infected with NeAL218 and cultured in RTMP for 13 days, but with only 7 or 10 days of doxycycline supplementation (scale bars 50μm). e. Bright field image of a representative hIA infected with NeAL218 and treated with RTMP media, probed by patch-clamp electrophysiology with its current profile (f) depicted in voltage-clamp mode. g. Current clamp recordings show the presence of hIA-derived iDANsNeAL218-RTMP capable of generating single action potential upon current injection (5, 20 and 30 pA). h. Vertical box plots, reflecting amplitudes of outward and inward currents in control (n=16) and NeAL218-infected hIAs cells with RTMP protocol (n=12). Whitney Rank Sum Test for outward currents p = 0,043; for inward currents p < 0,001. i. Pie chart depicting the fraction of hIA-derived iDANsNeAL218-RTMP generating single action potentials among the 12 cells probed * p<0.05 and ** p<0.01.
Supplementary Figure 3 Properties of hPA-derived control and iDANsNeAL218-RTMP cells
a. Efficient expression of GFP by hPAs in RTMP media at day 17. b. Uninfected hPAs do not become TH+ in RTMP media at day 17 (scale bar 50μm). c. Representative voltage-clamp (top) and current-clamp (bottom) recordings from control-RTMP hPAs cells. Control cells did not generate any action potential (AP). d. Comparison of gene expression by real-time q-PCR of hPAs treated with control-RTMP or NeAL218-RTMP at day 10. Data are mean ± s.d. FOXA2 was compared to untreated cells at day 0, as Foxa2 mRNA was undetectable in control-RTMP cells at day 10.
Supplementary Figure 4 Characterization of the 6-OHDA lesion model, transduction of the GFAP-tTA mice and reporter activity of the GFAP-tTa;SLC6A3cre/+;Rosa26RTomato mice.
a. Ventral midbrain cryosection of GFAP-tTA adult mice infected in the striatum with NeAL218 for 13 weeks (scale bar 50μm). Comparison of the right and left substantia nigra showing the effect of the 6-OHDA injection in the ipsilateral medial forebrain bundle. b. Striatal cryosections of GFAP-tTA adult mice 2 weeks after unilateral 6-OHDA (scale bar 100μm) showing loss of TH and increase in GFAP in the ipsilateral striatum. c. Striatal cryosections of GFAP-tTA adult mice infected with GFP for 2 weeks (scale bar 25μm). d. Striatal cryosections of GFAP-tTA adult mice infected with GFP for 13 weeks (scale bar 10μm). e. Striatal cryosections of GFAP- tTa;SLC6A3cre/+;Rosa26RTomato adult mice infected with GFP for 5 weeks (scale bar 10μm). f. Cryosection of adult GFAP-tTa;SLC6A3cre/+;Rosa26RTomato mice showing the functionality of the reporter mice in the intact contralateral side of 6-OHDA lesioned mice infected with GFP or NeAL218 for 5 weeks (scale bar 10μm).
Supplementary Figure 5 Characterization of in vivo iDAN reprogramming by NeAL218.
a. Diagram depicting am alternative strategy used to visualize in vivo reprogrammed mature iDANs: GFAP-tTA; SLC6A3Cre/+ mice and flex-tdTomato virus. b. Time-lapse microphotographs showing release of a fluorescent dopamine derivative, FFN 206, upon KCl-induced depolarization in acute brain slices of GFAP-tTA adult mice, 5 weeks after NeAL218 or GFP injection (scale bar 25μm). c-e. Electrophysiological recordings at 5 weeks showing NeAL218-induced SLC6A3Tomato+ cells unable to generate action potentials in voltage clamp mode (c: voltage clamp profile, n=19 out of 28) or able to generate single action potentials upon somatic current injection (n=4 out of 28 in d and e, showing voltage-clamp and current clamp profiles, respectively). f. Control GFP+ cells recorded in voltage-clamp configuration (polarization for 100 ms from -100 to + 50 mV, n=9; 2 examples are presented) showed outward voltage-gated currents but no inward voltage gated (sodium/calcium) currents. g. Net rotations after systemic amphetamine administration to control and 6-OHDA lesioned GFAP-tTA mice ± intrastriatatal GFP or NeAL218 for 5, 9 or 13 weeks. GFP n = 6; NeAL218, n = 7. Two Way RM ANOVA with time effect F(4,44) = 34,75; p < 0,0001 and subject matching F(11, 44) = 7,908; p < 0,0001 followed by Fisher’s comparison (for GFP: naïve vs lesion p = 0,0003; W5 vs lesion p = 0,0125; W9 vs lesion p = 0,0039 and W13 vs lesion p = 0,0122 and for NeAL218: naïve vs lesion p < 0,0001; W5 vs lesion p = 0,0813; W9 vs lesion p = 0,0011 and W13 vs lesion p = 0,0649).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–5 (PDF 1199 kb)
Rights and permissions
About this article
Cite this article
Rivetti di Val Cervo, P., Romanov, R., Spigolon, G. et al. Induction of functional dopamine neurons from human astrocytes in vitro and mouse astrocytes in a Parkinson's disease model. Nat Biotechnol 35, 444–452 (2017). https://doi.org/10.1038/nbt.3835
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nbt.3835