Abstract
Dopaminergic neurons in the ventral tegmental area (VTA) are well known for mediating the positive reinforcing effects of drugs of abuse. Here we identify in rodents and humans a population of VTA dopaminergic neurons expressing corticotropin-releasing factor (CRF). We provide further evidence in rodents that chronic nicotine exposure upregulates Crh mRNA (encoding CRF) in dopaminergic neurons of the posterior VTA, activates local CRF1 receptors and blocks nicotine-induced activation of transient GABAergic input to dopaminergic neurons. Local downregulation of Crh mRNA and specific pharmacological blockade of CRF1 receptors in the VTA reversed the effect of nicotine on GABAergic input to dopaminergic neurons, prevented the aversive effects of nicotine withdrawal and limited the escalation of nicotine intake. These results link the brain reward and stress systems in the same brain region to signaling of the negative motivational effects of nicotine withdrawal.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Volkow, N.D., Fowler, J.S., Wang, G.J., Baler, R. & Telang, F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology 56 (suppl. 1), 3–8 (2009).
Koob, G.F. & Le Moal, M. Addiction and the brain antireward system. Annu. Rev. Psychol. 59, 29–53 (2008).
Koob, G.F. & Bloom, F.E. Cellular and molecular mechanisms of drug dependence. Science 242, 715–723 (1988).
Koob, G.F. & Volkow, N.D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Sarnyai, Z., Shaham, Y. & Heinrichs, S.C. The role of corticotropin-releasing factor in drug addiction. Pharmacol. Rev. 53, 209–243 (2001).
Picciotto, M.R. & Kenny, P.J. Molecular mechanisms underlying behaviors related to nicotine addiction. Cold Spring Harb. Perspect. Med. 3, a012112 (2013).
Boyson, C.O., Miguel, T.T., Quadros, I.M., Debold, J.F. & Miczek, K.A. Prevention of social stress-escalated cocaine self-administration by CRF-R1 antagonist in the rat VTA. Psychopharmacology (Berl.) 218, 257–269 (2011).
Hahn, J., Hopf, F.W. & Bonci, A. Chronic cocaine enhances corticotropin-releasing factor-dependent potentiation of excitatory transmission in ventral tegmental area dopamine neurons. J. Neurosci. 29, 6535–6544 (2009).
Wanat, M.J., Bonci, A. & Phillips, P.E. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat. Neurosci. 16, 383–385 (2013).
Wang, B., You, Z.B., Rice, K.C. & Wise, R.A. Stress-induced relapse to cocaine seeking: roles for the CRF2 receptor and CRF-binding protein in the ventral tegmental area of the rat. Psychopharmacology (Berl.) 193, 283–294 (2007).
Wang, H.L. & Morales, M. Corticotropin-releasing factor binding protein within the ventral tegmental area is expressed in a subset of dopaminergic neurons. J. Comp. Neurol. 509, 302–318 (2008).
Blacktop, J.M. et al. Augmented cocaine seeking in response to stress or CRF delivered into the ventral tegmental area following long-access self-administration is mediated by CRF receptor type 1 but not CRF receptor type 2. J. Neurosci. 31, 11396–11403 (2011).
Vranjkovic, O., Gasser, P.J., Gerndt, C.H., Baker, D.A. & Mantsch, J.R. Stress-induced cocaine seeking requires a beta-2 adrenergic receptor-regulated pathway from the ventral bed nucleus of the stria terminalis that regulates CRF actions in the ventral tegmental area. J. Neurosci. 34, 12504–12514 (2014).
Grieder, T.E. et al. Dopaminergic signaling mediates the motivational response underlying the opponent motivational process to chronic but not acute nicotine. Neuropsychopharmacology 35, 943–954 (2010).
Grieder, T.E. et al. Phasic D1 and tonic D2 dopamine receptor signaling double dissociate the motivational effects of acute nicotine and chronic nicotine withdrawal. Proc. Natl. Acad. Sci. USA 109, 3101–3106 (2012).
Swanson, L.W., Sawchenko, P.E., Rivier, J. & Vale, W.W. Organization of ovine corticotropin-releasing factor immunoreactive cells and fibers in the rat brain: an immunohistochemical study. Neuroendocrinology 36, 165–186 (1983).
Tagliaferro, P. & Morales, M. Synapses between corticotropin-releasing factor-containing axon terminals and dopaminergic neurons in the ventral tegmental area are predominantly glutamatergic. J. Comp. Neurol. 506, 616–626 (2008).
Kozicz, T. et al. The Edinger-Westphal nucleus: a historical, structural, and functional perspective on a dichotomous terminology. J. Comp. Neurol. 519, 1413–1434 (2011).
Zhao-Shea, R. et al. Nicotine-mediated activation of dopaminergic neurons in distinct regions of the ventral tegmental area. Neuropsychopharmacology 36, 1021–1032 (2011).
Merlo Pich, E. et al. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 15, 5439–5447 (1995).
Funk, C.K., O'Dell, L.E., Crawford, E.F. & Koob, G.F. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 26, 11324–11332 (2006).
Tolu, S. et al. Co-activation of VTA DA and GABA neurons mediates nicotine reinforcement. Mol. Psychiatry 18, 382–393 (2013).
Hnasko, T.S., Hjelmstad, G.O., Fields, H.L. & Edwards, R.H. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J. Neurosci. 32, 15076–15085 (2012).
Nimitvilai, S., Arora, D.S., McElvain, M.A. & Brodie, M.S. Reversal of inhibition of putative dopaminergic neurons of the ventral tegmental area: interaction of GABA(B) and D2 receptors. Neuroscience 226, 29–39 (2012).
Doyon, W.M. et al. Nicotine decreases ethanol-induced dopamine signaling and increases self-administration via stress hormones. Neuron 79, 530–540 (2013).
Mansvelder, H.D., Keath, J.R. & McGehee, D.S. Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron 33, 905–919 (2002).
George, O. et al. CRF–CRF1 system activation mediates withdrawal-induced increases in nicotine self-administration in nicotine-dependent rats. Proc. Natl. Acad. Sci. USA 104, 17198–17203 (2007).
Cohen, A. et al. Extended access to nicotine leads to a CRF1 receptor dependent increase in anxiety-like behavior and hyperalgesia in rats. Addict. Biol. 10.1111/adb.12077 (2013).
Cohen, A., Koob, G.F. & George, O. Robust escalation of nicotine intake with extended access to nicotine self-administration and intermittent periods of abstinence. Neuropsychopharmacology 37, 2153–2160 (2012).
Ludwig, M. & Leng, G. Dendritic peptide release and peptide-dependent behaviours. Nat. Rev. Neurosci. 7, 126–136 (2006).
Son, S.J. et al. Dendritic peptide release mediates interpopulation crosstalk between neurosecretory and preautonomic networks. Neuron 78, 1036–1049 (2013).
Treweek, J.B., Jaferi, A., Colago, E.E., Zhou, P. & Pickel, V.M. Electron microscopic localization of corticotropin-releasing factor (CRF) and CRF receptor in rat and mouse central nucleus of the amygdala. J. Comp. Neurol. 512, 323–335 (2009).
Wang, B. et al. Cocaine experience establishes control of midbrain glutamate and dopamine by corticotropin-releasing factor: a role in stress-induced relapse to drug seeking. J. Neurosci. 25, 5389–5396 (2005).
Marcinkiewcz, C.A. et al. Corticotropin-releasing factor within the central nucleus of the amygdala and the nucleus accumbens shell mediates the negative affective state of nicotine withdrawal in rats. Neuropsychopharmacology 34, 1743–1752 (2009).
Baiamonte, B.A. et al. Nicotine dependence produces hyperalgesia: role of corticotropin-releasing factor-1 receptors (CRF1Rs) in the central amygdala (CeA). Neuropharmacology 77, 217–223 (2014).
Torres, O.V., Gentil, L.G., Natividad, L.A., Carcoba, L.M. & O'Dell, L.E. Behavioral, biochemical, and molecular indices of stress are enhanced in female versus male rats experiencing nicotine withdrawal. Front. Psychiatry 4, 38 (2013).
Zhang, L., Dong, Y., Doyon, W.M. & Dani, J.A. Withdrawal from chronic nicotine exposure alters dopamine signaling dynamics in the nucleus accumbens. Biol. Psychiatry 71, 184–191 (2012).
Lodge, D.J. & Grace, A.A. Acute and chronic corticotropin-releasing factor 1 receptor blockade inhibits cocaine-induced dopamine release: correlation with dopamine neuron activity. J. Pharmacol. Exp. Ther. 314, 201–206 (2005).
Willuhn, I., Burgeno, L.M., Groblewski, P.A. & Phillips, P.E. Excessive cocaine use results from decreased phasic dopamine signaling in the striatum. Nat. Neurosci. 17, 704–709 (2014).
Kalivas, P.W., Duffy, P. & Latimer, L.G. Neurochemical and behavioral effects of corticotropin-releasing factor in the ventral tegmental area of the rat. J. Pharmacol. Exp. Ther. 242, 757–763 (1987).
van Zessen, R., Phillips, J.L., Budygin, E.A. & Stuber, G.D. Activation of VTA GABA neurons disrupts reward consumption. Neuron 73, 1184–1194 (2012).
Tan, K.R. et al. GABA neurons of the VTA drive conditioned place aversion. Neuron 73, 1173–1183 (2012).
Roberto, M. et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric acid release plays a key role in alcohol dependence. Biol. Psychiatry 67, 831–839 (2010).
Nader, K. & van der Kooy, D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J. Neurosci. 17, 383–390 (1997).
Vargas-Perez, H. et al. Ventral tegmental area BDNF induces an opiate-dependent like reward state in naive rats. Science 324, 1732–1734 (2009).
Laviolette, S.R., Alexson, T.O. & van der Kooy, D. Lesions of the tegmental pedunculopontine nucleus block the rewarding effects and reveal the aversive effects of nicotine in the ventral tegmental area. J. Neurosci. 22, 8653–8660 (2002).
Steffensen, S.C. et al. Contingent and non-contingent effects of heroin on mu-opioid receptor-containing ventral tegmental area GABA neurons. Exp. Neurol. 202, 139–151 (2006).
Volkow, N.D., Fowler, J.S., Wang, G.J., Swanson, J.M. & Telang, F. Dopamine in drug abuse and addiction: results of imaging studies and treatment implications. Arch. Neurol. 64, 1575–1579 (2007).
Koob, G.F. et al. Addiction as a stress surfeit disorder. Neuropharmacology 76 (pt. B), 370–382 (2014).
Paxinos, G. & Franklin, K. B. J. The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego, 1997).
Richardson, H.N. et al. MPZP: a novel small molecule corticotropin-releasing factor type 1 receptor (CRF1) antagonist. Pharmacol. Biochem. Behav. 88, 497–510 (2008).
Darcq, E. et al. RSK2 signaling in brain habenula contributes to place aversion learning. Learn. Mem. 18, 574–578 (2011).
Zolotukhin, S. et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods 28, 158–167 (2002).
Acknowledgements
The authors thank M. Brennan, B. Takabe, C. Arias and the University of Toronto Division of Comparative Medicine staff for technical assistance and M. Arends for editorial assistance. The authors would also like to thank R. Nagra and J. Riehl and the UCLA Brain Bank (The Human Brain and Spinal Fluid Resource Center) for providing the human samples. This work was supported by the Canadian Institutes of Health Research, US National Institute on Drug Abuse (DA023597, DA035371 and DA031566), US National Institute on Alcohol Abuse and Alcoholism (AA021491, AA015566, F32 AA020430, AA006420, AA016658, AA021667 and INIA AA013498), Tobacco-Related Disease Research Program (12RT-0099), US National Institute of Diabetes and Digestive and Kidney Diseases (DK026741) and the Clayton Medical Research Foundation.
Author information
Authors and Affiliations
Contributions
T.E.G. and O.G. designed the experiments. T.E.G., H.V.-P., M.C., J.E.S. and G.M.-B. performed minipump, cannulation and viral vector surgeries. M.R. and M.A.H. performed the electrophysiology experiments. T.E.G. performed place conditioning and open field testing. A.C. performed self-administration experiments. C.C., L.A.T. and P.E.S. performed ISH. C.C. and E.C. performed double ISH and immunohistochemistry. T.E.G., V.R.-C., P.P.S., A.R.T. and L.C. performed molecular studies. J.F. and E.C. performed immunohistochemistry. C.C., P.K. and B.L.K. supplied viral vectors. T.E.G. and O.G. analyzed the data. T.E.G., C.C., G.F.K., D.v.d.K. and O.G. wrote the paper. All of the authors discussed the results and read the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Integrated supplementary information
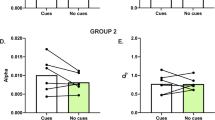
Supplementary Figure 1 Crh mRNA relative expression in the CeA, VTA and PVN.
Crh mRNA levels in the CeA, VTA, and PVN. Crh mRNA levels in the CeA, VTA, and PVN relative to the housekeeping gene GAPDH in mice chronically exposed to saline, measured by RT-PCR (n = 13 or 14 per group). Lower levels of Crh mRNA were observed in the VTA compared with the CeA and PVN (*p < 0.05).
Supplementary Figure 2 Number of Crh mRNA–positive neurons in the VTA
Number of CRF neurons per section in the VTA (bregma range: -2.92 to -3.88) in saline minipump, saline minipump + acute nicotine (1.5 mg/kg), nicotine minipump (12 days), and nicotine minipump and withdrawn (8 h) groups of mice (n = 6 per group). No difference was observed between groups. Data in Fig. 2 in the manuscript represents combined data from the four groups.
Supplementary Figure 3 Urocortin in situ hybridization in the VTA and Edinger-Westphal nucleus.
Notice the lack of urocortin neurons in the VTA and the prominent population of urocortin-positive neurons in the Edinger-Westphal nucleus. This result was repeated on 6 naïve and 6 nicotine dependent mice. Urocortin neurons were never observed in the VTA.
Supplementary Figure 4 CRF immunodensity in the VTA, CeA, PVN and IPN in nicotine-dependent and withdrawn mice.
Quantification of immunodensity in the aVTA and pVTA (a), PVN (b), CeA (c) and IPN (d) in nondependent mice treated with saline (Sal), nicotine-dependent mice (Nic) and nicotine-dependent and -withdrawn mice (WD; n = 12-26 mice per group). e. Example of CRF-immunostained neuropils in the CeA showing CRF varicosities in a transversal fiber. Densitometry analysis revealed that both nicotine dependence and withdrawal from chronic nicotine decreased CRF peptide density compared with saline-treated mice in the pVTA (F2,31 = 4.40, p = 0.02; Fig. S4a), interpeduncular nucleus (IPN: F2,49 = 4.24, p = 0.020; Fig S4d), and CeA (F2,67 = 3.15, p = 0.049; Fig. S4c) but not aVTA (F2,23 = 0.03, p = 0.97; Fig. S4a) or PVN (F2,21 = 0.75, p = 0.48; Fig. S4b). Note that a different number of sections were used for the different brain regions resulting in a different degree of freedom. This experiment was performed with 1 cohort.
Supplementary Figure 5 Validation of the AAV2-shCRH viral vector.
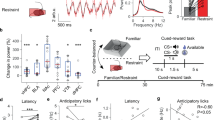
a, Timeline of viral vector infusion and behavioral experiments in mice. b, DNA construct used to produce vectors for CRF silencing (AAV2-shCRH) and control vectors (AAV2-shSCR). ITR, inverted terminal repeat; CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein; hGH polyA, human growth hormone polyadenylation signal. c, Representative images of VTA Crh mRNA-containing cells taken at 20× magnification. d, Number of Crh mRNA-containing cells per section. Quantification of Crh-positive neurons restricted to GFP-positive areas in the pVTA revealed significant downregulation (-26%) of the number of Crh mRNA-containing cells in AAV2-shCRH mice compared with AAV2-shSCR mice (t12 = 2.7, p = 0.020). AAV2-shCRH also decreased the total number of CRF neurons in the entire VTA (including the region without GFP) by 18% (t12 = 2.4, p = 0.033). Notably, the mice were sacrificed approximately 4 weeks into withdrawal after the end of behavioral testing, demonstrating that the upregulation of Crh mRNA in nicotine-withdrawn mice and CRF silencing by the viral vector were both long-lasting. This experiment was performed with 1 cohort.
Supplementary Figure 6 Electrophysiological recording of VTA DA neurons.
a, Representative trace of a recorded VTA DA neuron. b, Definition of DA neuron action potential characteristics. c, Characterization of VTA dopamine neurons from naive (n = 8 cells), nicotine-dependent AAV2-shSCR (n = 11 cells), and nicotine-dependent AAV2-shCRH (n = 12 cells) mice (n = 3 naïve, n = 4 nicotine-dependent AAV2-shSCR, n = 4 nicotine-dependent AAV2-shCRH). This experiment was performed with 1 cohort.
Supplementary Figure 7 Comparison of confirmed TH-positive VTA cells with all cells examined.
a, Effects of acute nicotine in naive mice on sIPSC frequency in all VTA cells examined (left; *p = 0.0015 by paired t-test) compared with TH+-only VTA cells (right; *p = 0.0051). The total number of cells for each condition is indicated in parentheses. b, Average change in sIPSC frequency in all VTA cells examined (left; *p = 0.0026, one-sample t-test; #p = 0.0003, unpaired t-test) compared with TH+-only VTA cells (right; *p = 0.0128, one-sample t-test; #p = 0.0005, unpaired t-test). Exact t and p values noted under each condition. Total number of cells for each condition indicated in parentheses.
Supplementary Figure 8 Anatomical specificity of AAV2-shCRH VTA injections.
Mice with AAV2-shCRH injections outside of the VTA (n = 7) showed an aversive motivational response to nicotine withdrawal that was not significantly different from mice injected with AAV2-shSCR (n = 11).
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–8 and Supplementary Table 1 (PDF 2041 kb)
Supplementary Methods Checklist
(PDF 416 kb)
Rights and permissions
About this article
Cite this article
Grieder, T., Herman, M., Contet, C. et al. VTA CRF neurons mediate the aversive effects of nicotine withdrawal and promote intake escalation. Nat Neurosci 17, 1751–1758 (2014). https://doi.org/10.1038/nn.3872
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.3872
This article is cited by
-
Paternal cocaine-seeking motivation defines offspring’s vulnerability to addiction by down-regulating GABAergic GABRG3 in the ventral tegmental area
Translational Psychiatry (2024)
-
How nicotine withdrawal symptoms fight each other: interpeduncular GABA neuron activity dynamically controls negative affect vs. coping behavior
Neuropsychopharmacology (2022)
-
PSD-93 up-regulates the synaptic activity of corticotropin-releasing hormone neurons in the paraventricular nucleus in depression
Acta Neuropathologica (2021)
-
Nicotine preference and affective behavior of Cd81 knockout mice
Psychopharmacology (2021)
-
Flavor additives facilitate oral self-administration of nicotine solution in mice
Psychopharmacology (2021)